A multicentre single-arm phase II trial assessing the safety and efficacy of first-line osimertinib and locally ablative radiotherapy in patients with synchronous oligo-metastatic EGFR-mutant non-small cell lung cancer.
STEREO: Osimertinib and locally ablative radiotherapy in patients with synchronous oligo-metastatic EGFR mutant NSCLC
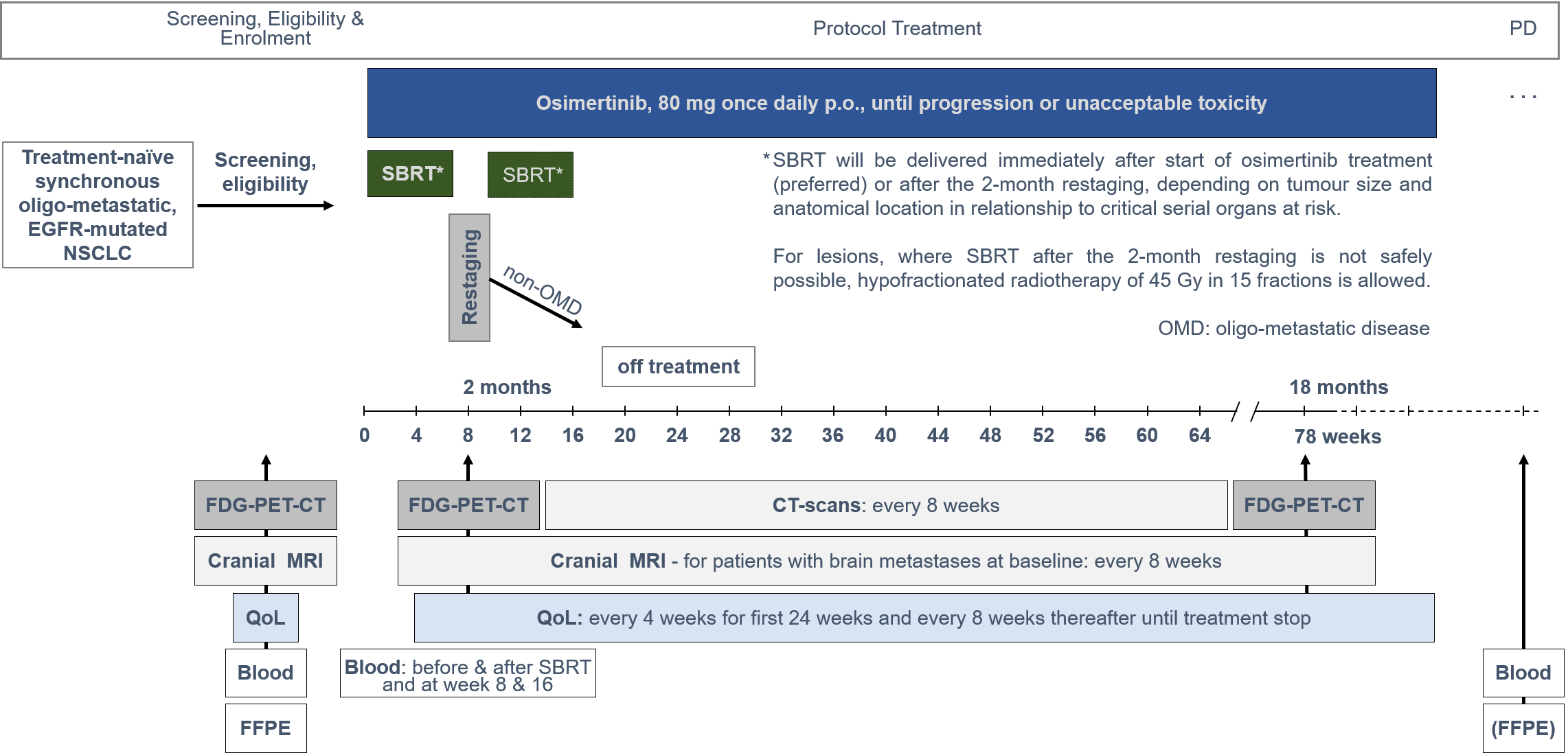
The trial aimed to explore the safety and efficacy of osimertinib treatment and locally ablative radiotherapy to all cancer sites, in patients with synchronous oligo-metastatic EGFR-mutant NSCLC.
Trial Scheme