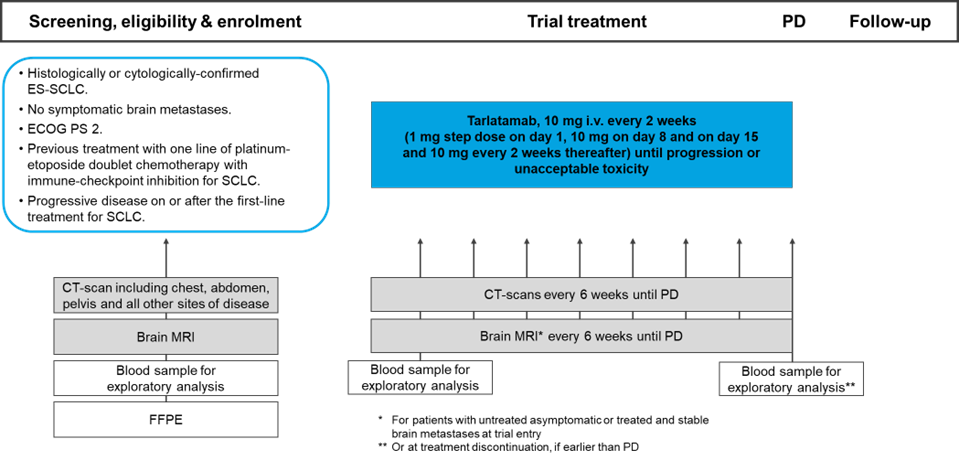
A multicentre phase II trial of tarlatamab in patients with pretreated extensive-stage small cell lung cancer (ES-SCLC) and ECOG PS 2
The aim of the study is to evaluate the clinical efficacy of tarlatamab in patients with extensive-stage small cell lung cancer (ES-SCLC) and ECOG PS 2, who have relapsed on or after previous standard platinum-etoposide doublet chemotherapy and immune-checkpoint inhibition therapy. The primary endpoint is the overall survival rate at 12 months.
Trial Scheme